For those of you within the zebra community, you are probably already aware of an association between autism (and other neurodevelopmental conditions like ADHD) and Ehlers-Danlos syndrome (EDS)/hypermobility spectrum disorders (HSD). But what may not be clear is how these different conditions are related.
It’s early days and the data is tenuous so far, but we do have some clues as to why these conditions either co-occur or aggregate within the same families. While we don’t currently know whether dysfunctional or dysregulated connective tissue itself is directly influencing brain development (a possibility), we suspect that chronic maternal inflammation (e.g., mast cell activation syndrome or MCAS) is playing a role in autism development in these kids.
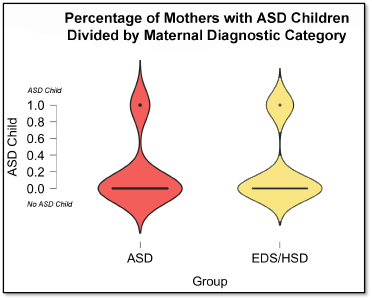
In one of our studies [1], we found that moms with EDS/HSD were equally as likely to report having autistic kids as were moms with autism (but without EDS/HSD). This suggests that EDS/HSD is as heritable for autism as autism itself is– if that makes any sense!
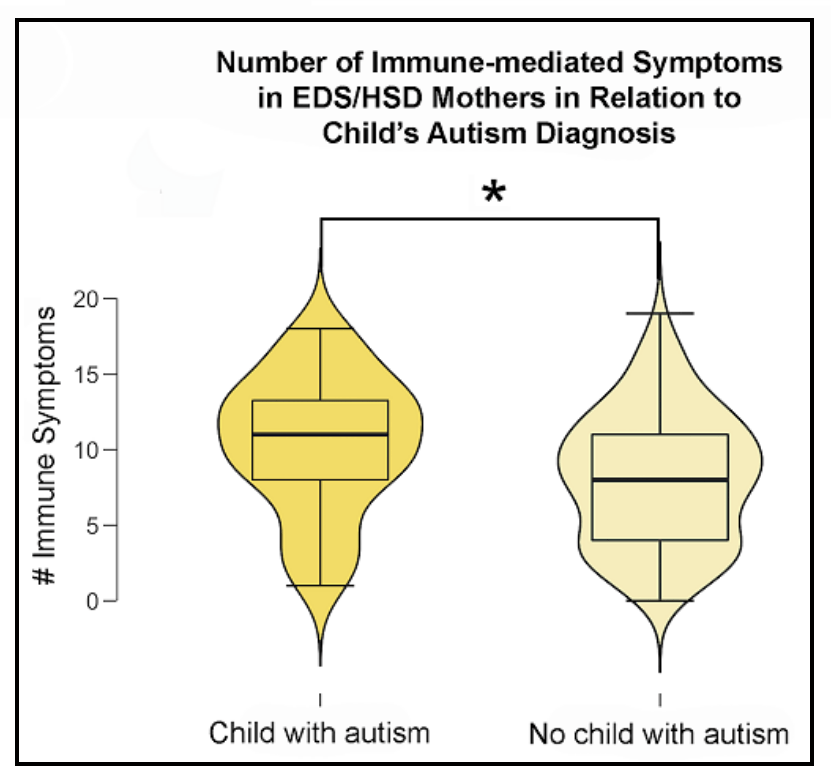
We also found that within the EDS/HSD moms group, moms who had autistic kids were significantly more likely to report having more serious immune problems, suggesting that the maternal immune system could have an influencing role during embryonic brain development and increasing the likelihood of autism.
You may be wondering what in the world a mother’s immune disorder could possibly have to do with the brain development of her child.
Interestingly, there’s been a lot of research, both human studies and animal models, that provide links between the two. We know, for instance, that moms who experience infection during pregnancy are more likely to have kids that go on to develop a variety of neurodevelopmental conditions like autism and schizophrenia. We also know that mothers with autoimmune disorders are also more likely have kids with these conditions. The presence of certain maternal brain-specific autoantibodies also seems to share links with autism, although it’s not currently certain whether the autoantibodies are playing a direct role in brain development but one primate study suggests that could be the case [2]. And, finally, other animal models of maternal immune activation (MIA) consistently exhibit autistic-like behaviors (at least as far as one can say “A mouse has autism.”) [most of these studies are reviewed here].
So, it seems clear that maternal immune disorders or infection can increase the likelihood that an exposed embryo or fetus will go on to develop autism or some other type of neurodevelopmental condition. But how does that happen?
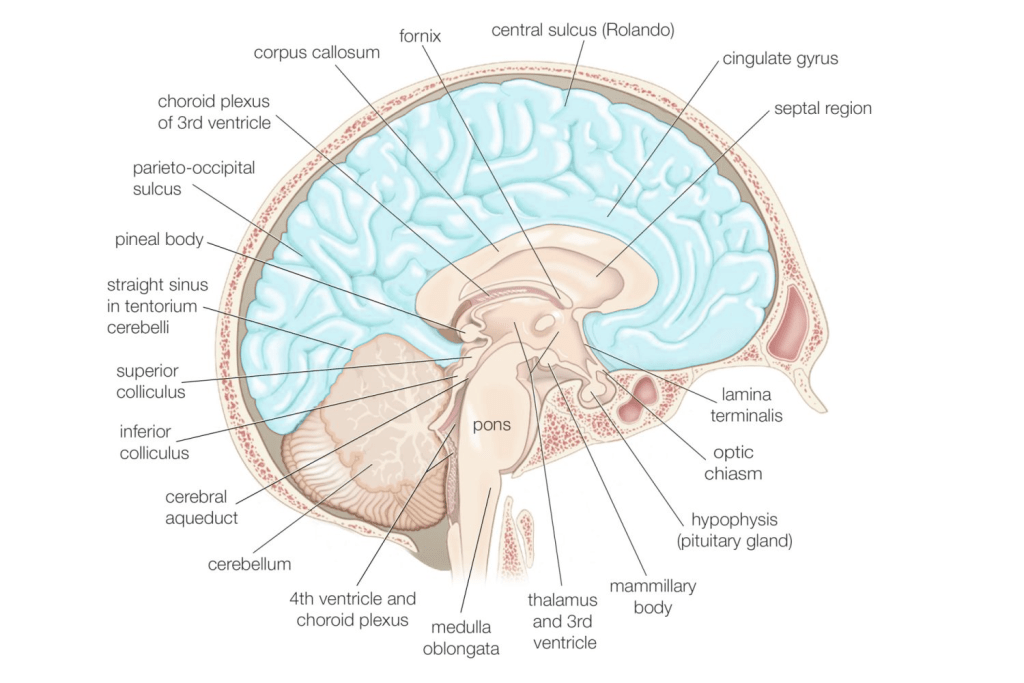
In conditions like autism and schizophrenia, there’s been a lot of emphasis placed on the roles of a group of cells known as “inhibitory interneurons.” For our purposes here, we’re going to focus on these cells within the neocortex (the teal-highlighted structure in the image below) because many of the symptoms most characteristic of autism are largely rooted in this vital tissue.
Inhibitory interneurons form networks around excitatory (pyramidal) neurons and work by– you guessed it– inhibiting the activity of these excitatory cells. Some types of interneurons act like on/off switches, regulating pyramidal cell activity completely, while others instead fine-tune that activity in more nuanced ways.
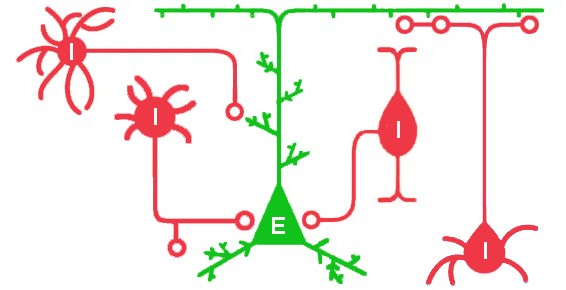
During early brain development, each of these cell populations must travel from the place of their birth to their final destinations. In the case of excitatory cells, that’s a relatively short jaunt from the area surrounding the cerebral ventricles up into the developing neocortex immediately overhead. However, in the case of inhibitory interneurons, that means traveling all the way from the underside of the developing brain up to the top (see arrows below), where the cortex is developing.
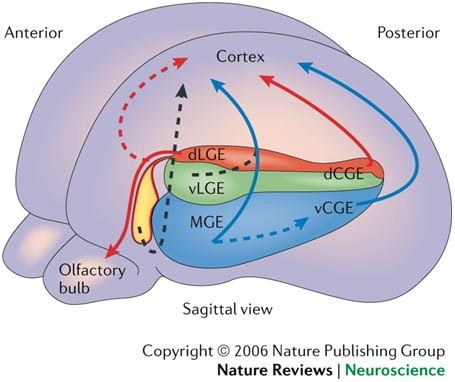
Unfortunately, that means that a lot can happen along the way to disturb an interneuron’s travels. One of those things is the mother’s immune system. Animal models of MIA indicate that immune activation within the mother leads to characteristic changes in gene expression, some of which directly target these inhibitory cells. Specifically, immune activation triggers a stress response within the developing brain, which appears to waylay the developmental process, downregulating many important proteins necessary for development (and migration) of these cells [3].
In the end, the more severe the disruption, likely the fewer of these interneurons actually make it to their final destinations and those that do may well carry with them permanent effects of their exposure. Similar disruptions in migration can occur even with just maternal stress, unrelated to immune activation [4]. Ultimately, that means that fewer interneurons (or even malfunctioning interneurons) may form those all-important networks surrounding excitatory neurons, potentially leading to imbalances in the excitatory-inhibitory network (with an emphasis now on excitation) and many of the characteristic symptoms of autism [5].
Although I’ve primarily focused on inhibitory interneurons in this blog, it’s entirely possible and probable that MIA also affects the development of excitatory neurons as well. However, due to shorter migratory ranges, they may be less susceptible to interruptions. Therefore, autism is undoubtedly due to a number of factors as a result of MIA, but inhibitory interneurons are likely a major piece of that puzzle.

Thank you for this thought provoking post. Some researchers and clinicians report that the severity of autism is worse with each subsequent pregnancy. Could Feto-maternal microchimerism play role? I am also wondering about multi generational autism. Could inflammation in the grandmother cause autism in the daughter, who in turn has inflammation in her pregnancy? Although like so many other things, there are so many factors, genetic, development and environmental, that could come into play.
Hi, cyberjennifer. Unfortunately, I don’t have the knowledge-based to address issues about microchimerism. However, there are suggestions that some animal models like C. elegans exhibit multigenerational “learning” (i.e., epigenetic) for specific pathogens. So, while there probably isn’t much evidence by way of humans, it’s theoretically feasible.